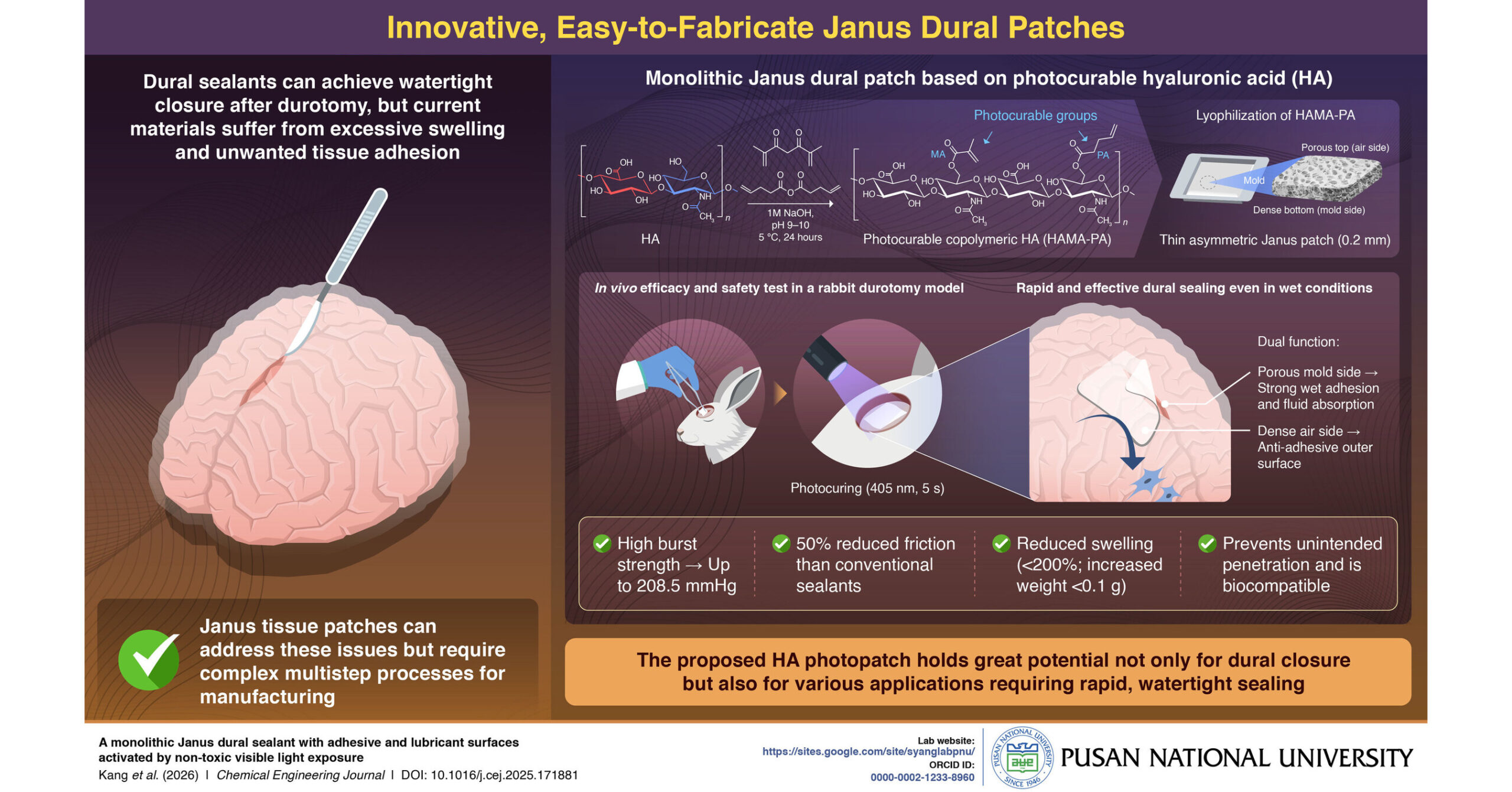
SAN FRANCISCO, Jan. 16, 2026 /PRNewswire/ — The 44th J.P.Morgan Healthcare Conference (JPMHC) was successfully held in San Francisco, the United States, from January 12 to 15. On January 15 (PST), Dr. Jason Zhu, Executive Director and Chief Executive Officer of Shanghai Henlius Biotech, Inc. (2696.HK), delivered a keynote presentation outlining Henlius’ “Globalisation 2.0” strategy, diversified innovation pipeline, and its mid-to-long-term development blueprint.
The Company’s international business continues to grow at a rapid pace. Looking ahead to 2030, Henlius anticipates to launch more than 20 products globally, including over 15 products in the U.S. and European markets.
Dr. Zhu highlighted Henlius’ continuous growth trajectory and phased achievements under its “Globalisation 2.0” strategy:
“With multiple products approved successively in Europe and the United States, and an increasingly mature integrated global operating model, our international business has maintained strong growth momentum. Leveraging our integrated R&D, regulatory and manufacturing capabilities, together with an increasingly mature global clinical and commercialisation network, we have established a systematic capacity to continuously deliver innovative assets worldwide. Over the next five years, stable cash flows from our biosimilar portfolio will further support innovation investment, enabling the advancement of more differentiated molecules, including ADCs, multi-Abs and TCEs, into global markets and building a sustainable, replicable globalisation growth model.”
Henlius also outlined clear timelines and development plans for its key innovation assets in 2026, including serplulimab (trade name: Hetronifly® in Europe), an anti-PD-1 mAb; HLX22, a novel epitope anti-HER2 mAb; HLX43, a PD-L1 ADC; and HLX07, an anti-EGFR mAb.
At present, the Company’s preclinical asset portfolio spans multiple molecular modalities, including antibodies, multispecific TCEs, ADCs, fusion proteins and small molecules, with a primary focus on solid tumors. Its differentiated development strategy targets both established and emerging targets such as PD-(L)1, DLL3, B7-H3, HER2, EGFR, c-Met and KAT6A/B. The portfolio comprises a balanced mix of potential FIC and BIC candidates, as well as fast-follow programs with higher clinical and commercialisation certainty, laying a solid foundation for the sustained advancement of the mid- to long-term clinical pipeline. By proactively structuring its preclinical portfolio to encompass diverse innovation profiles including FIC, BIC and fast-follow programs, the company has established a tiered R&D architecture that balances frontier innovation with development efficiency and risk management, thereby supporting the continuous progression of its innovation pipeline.


Source link












Leave a Reply