The Challenge: Fragmented Data, Complex Trials
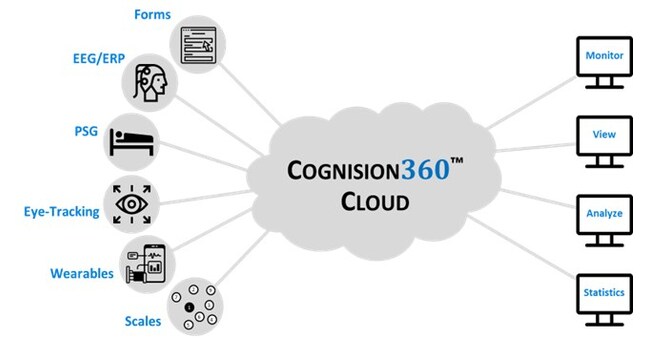
Multi-site CNS clinical trials have long struggled with operational complexity. Researchers juggle disparate systems for EEG, sleep studies, eye tracking, wearable sensors, and cognitive assessments—each generating data in different formats, requiring manual transfers, and creating opportunities for errors that can compromise study integrity. Cognision360™ changes this paradigm. One Platform, Multiple Data Streams, Zero Compromise
The platform brings together electroencephalography (EEG), event-related potentials (ERP), polysomnography (PSG), eye tracking, wearable sensors, cognitive assessments, and electronic clinical outcome assessments (eCOA, ePRO, and eObsRO) under one roof. What makes this significant? Every data point captured through Cognision360™ is automatically tagged with comprehensive study metadata—subject ID, site location, treatment arm, visit timepoint—from the moment of collection. This eliminates the manual data transcription and file transfers that plague traditional approaches, dramatically reducing errors and accelerating the path to database lock. “We’re seeing sponsors invest millions in sophisticated biomarker strategies, only to lose weeks or months wrestling with data integration issues,” said Dr. Igor Korolev, Vice President of Neuroscience and Digital Health at Cognision. “Cognision360™ provides an end-to-end framework that ensures multi-modal neurobiomarker data meets regulatory expectations for consistency and integrity from day one.” Built for Real-World Clinical Operations
The platform’s design reflects input from the full spectrum of trial stakeholders:
In an era of AI-driven analysis tools, Cognision360™ takes a deliberately different approach. “The platform employs deterministic, rules-based analytical methods with expert-defined data relationships rather than non-transparent artificial intelligence models,” explained K.C. Fadem, Chief Technology Officer. “For regulatory submissions, transparency, reproducibility, and traceability aren’t nice-to-haves—they’re requirements. Our approach is designed with that reality in mind.” Enables FDA’s New Bayesian Trial Guidelines
Cognision360™ enables rapid data acquisition, quality monitoring, analysis, and statistics through an automated, pipelined workflow that empowers sponsors to implement Bayesian adaptive trial designs consistent with the new FDA guidance. Enterprise-Grade Infrastructure, Research-Grade Flexibility
Hosted in a validated Microsoft Azure environment with role-based single sign-on, Cognision360™ meets enterprise security standards while maintaining the flexibility researchers need. An extensive API supports integration with existing clinical trial management systems, while built-in eCRF capabilities capture session-level metadata without adding separate systems. Study data remains continuously accessible through secure web interfaces for remote monitoring and analysis by authorized users—critical for decentralized trial models and real-time decision-making. At any stage of a study, data can be exported in formats ready for regulatory submissions, streamlining the transition from data collection to filing. About Cognision
Cognision delivers advanced neurophysiological biomarker services for clinical trials, specializing in EEG, ERP, PSG, eye-tracking, and cognitive testing. The company provides fully managed solutions—from protocol consultation through data analytics—ensuring consistency, accuracy, and regulatory compliance in pharmaceutical trials. FOR MEDIA INQUIRIES:
Nathan Alvarado
Director of Business Development [email protected] www.cognision.com
502-561-9040 x7005 SOURCE Cognision
Multi-site CNS clinical trials have long struggled with operational complexity. Researchers juggle disparate systems for EEG, sleep studies, eye tracking, wearable sensors, and cognitive assessments—each generating data in different formats, requiring manual transfers, and creating opportunities for errors that can compromise study integrity. Cognision360™ changes this paradigm. One Platform, Multiple Data Streams, Zero Compromise
The platform brings together electroencephalography (EEG), event-related potentials (ERP), polysomnography (PSG), eye tracking, wearable sensors, cognitive assessments, and electronic clinical outcome assessments (eCOA, ePRO, and eObsRO) under one roof. What makes this significant? Every data point captured through Cognision360™ is automatically tagged with comprehensive study metadata—subject ID, site location, treatment arm, visit timepoint—from the moment of collection. This eliminates the manual data transcription and file transfers that plague traditional approaches, dramatically reducing errors and accelerating the path to database lock. “We’re seeing sponsors invest millions in sophisticated biomarker strategies, only to lose weeks or months wrestling with data integration issues,” said Dr. Igor Korolev, Vice President of Neuroscience and Digital Health at Cognision. “Cognision360™ provides an end-to-end framework that ensures multi-modal neurobiomarker data meets regulatory expectations for consistency and integrity from day one.” Built for Real-World Clinical Operations
The platform’s design reflects input from the full spectrum of trial stakeholders:
- Sites conduct protocol-defined assessments efficiently using intuitive, touch-based mobile interfaces.
- Monitors benefit from automated quality control and pre-specified processing pipelines that standardize handling of complex neurobiological datasets.
- Sponsors access advanced visualization and statistical monitoring dashboards through traditional web interfaces, enabling near real-time tracking of biomarker trends and endpoints.
In an era of AI-driven analysis tools, Cognision360™ takes a deliberately different approach. “The platform employs deterministic, rules-based analytical methods with expert-defined data relationships rather than non-transparent artificial intelligence models,” explained K.C. Fadem, Chief Technology Officer. “For regulatory submissions, transparency, reproducibility, and traceability aren’t nice-to-haves—they’re requirements. Our approach is designed with that reality in mind.” Enables FDA’s New Bayesian Trial Guidelines
Cognision360™ enables rapid data acquisition, quality monitoring, analysis, and statistics through an automated, pipelined workflow that empowers sponsors to implement Bayesian adaptive trial designs consistent with the new FDA guidance. Enterprise-Grade Infrastructure, Research-Grade Flexibility
Hosted in a validated Microsoft Azure environment with role-based single sign-on, Cognision360™ meets enterprise security standards while maintaining the flexibility researchers need. An extensive API supports integration with existing clinical trial management systems, while built-in eCRF capabilities capture session-level metadata without adding separate systems. Study data remains continuously accessible through secure web interfaces for remote monitoring and analysis by authorized users—critical for decentralized trial models and real-time decision-making. At any stage of a study, data can be exported in formats ready for regulatory submissions, streamlining the transition from data collection to filing. About Cognision
Cognision delivers advanced neurophysiological biomarker services for clinical trials, specializing in EEG, ERP, PSG, eye-tracking, and cognitive testing. The company provides fully managed solutions—from protocol consultation through data analytics—ensuring consistency, accuracy, and regulatory compliance in pharmaceutical trials. FOR MEDIA INQUIRIES:
Nathan Alvarado
Director of Business Development [email protected] www.cognision.com
502-561-9040 x7005 SOURCE Cognision

Source link













Leave a Reply