VANCOUVER, BC, Feb. 9, 2026 /PRNewswire/ — WAT Medical is proud to announce the publication of a landmark randomized clinical trial in JAMA Surgery, one of the world’s most reputable and high-impact surgical journals. The study confirms that the EmeTerm wristband — a wearable device utilizing transcutaneous acupoint electrical stimulation — provides significantly better control of moderate to severe postoperative nausea and vomiting (PONV) compared to metoclopramide, a widely used antiemetic medication.
The patient-blinded, multicenter randomized clinical trial enrolled 232 female patients who developed moderate to severe PONV (numerical rating score [NRS] ≥4) following thyroidectomy or anterior cervical surgery under general anesthesia. Conducted across four leading hospitals, participants were randomized 1:1 to receive either active transcutaneous electrical acupoint stimulation (TEAS) via the EmeTerm wristband targeting the median nerve at acupoint PC6, or a control intervention consisting of a sham device combined with intravenous metoclopramide.
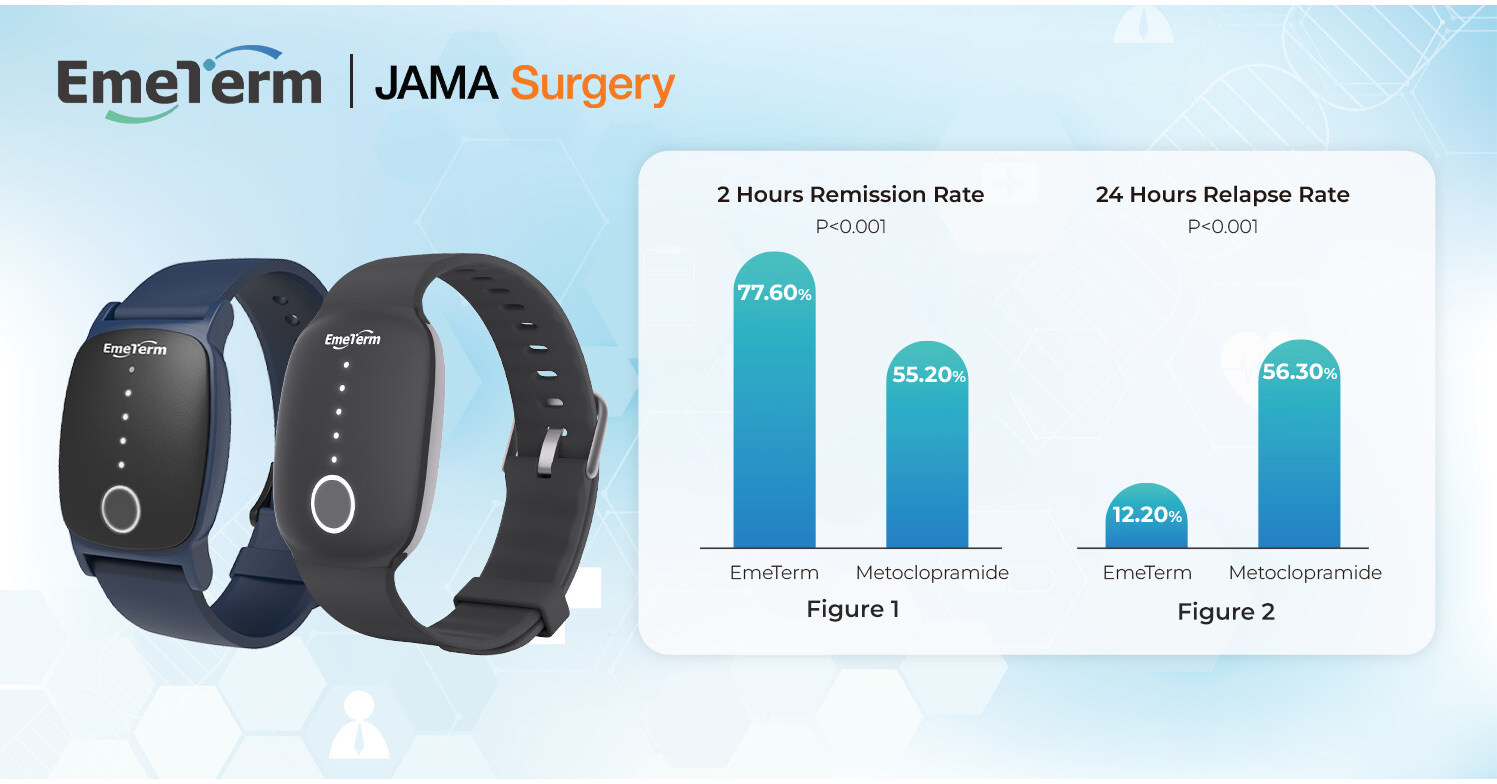
The primary endpoint was the 2-hour remission rate of PONV, while secondary endpoints included 24-hour relapse rates and crossover responses.

Key Findings
- 2-Hour Remission Rate: EmeTerm significantly improved PONV remission compared with metoclopramide (77.6% vs 55.2%, P < 0.001) (Figure 1).
- 24-Hour Relapse Rate: Patients treated with EmeTerm experienced significantly lower relapse rates than those treated with metoclopramide (12.2% vs 56.3%, P < 0.001) (Figure 2).
- Safety: No device-related serious adverse events were reported.
- Zheng D, Ding P, Gong M, et al. Transcutaneous Electrical Acupoint Stimulation vs Metoclopramide for Moderate to Severe Postoperative Nausea and Vomiting: A Randomized Clinical Trial. JAMA Surg. Published online January 28, 2026. doi:10.1001/jamasurg.2025.6394
- www.emeterm.com
- www.watmedical.com

Source link













Leave a Reply