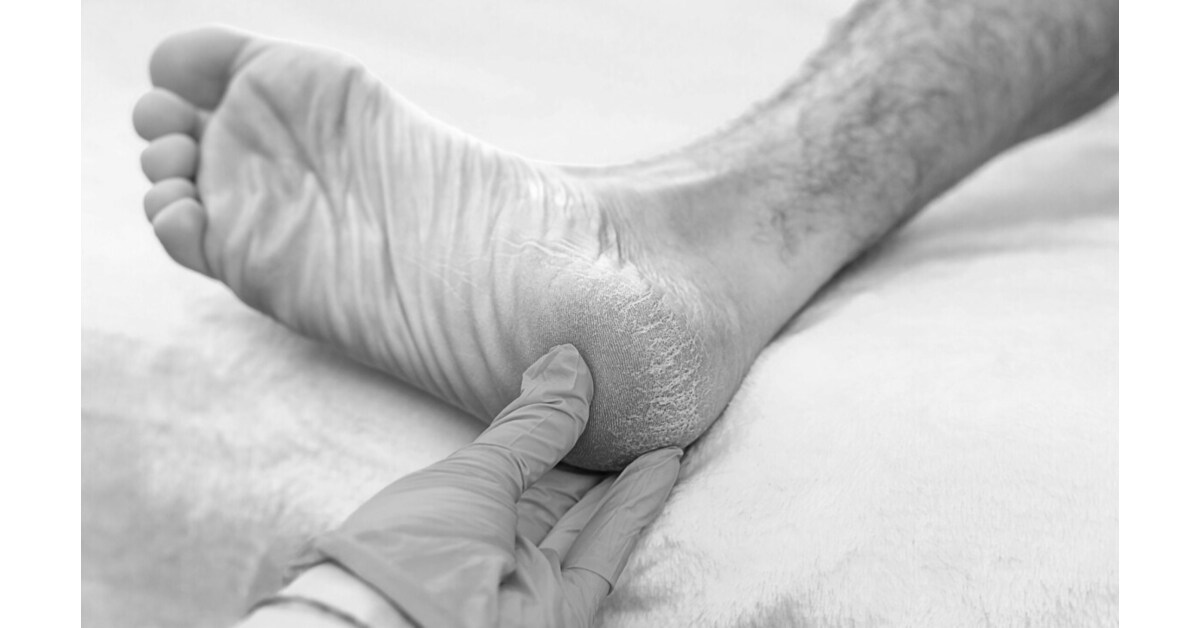
PENSACOLA, Fla., Feb. 4, 2026 /PRNewswire/ — Regenative Labs, in collaboration with Babak Baravarian, DPM, Director of Foot & Ankle Research at University Foot & Ankle Institute, today announced the publication of a new observational case series examining the use of Wharton’s jelly connective tissue allografts in patients with plantar fascia defects associated to plantar fasciopathy.
The study, titled “Wharton’s Jelly Tissue Allografts for Tearing in the Plantar Fascia: A Case Series,” was published on September 24, 2025, in Biomedicines, a peer-reviewed, open-access medical journal. The case series reports outcomes from nine patients with plantar fasciopathy based defects who received a single ultrasound-guided application of a collagen-rich Wharton’s jelly connective tissue allograft and were followed for 90 days.
Plantar fasciopathy—commonly referred to as plantar fasciitis—characterized by microtearing of the plantar fascia that can significantly impact mobility and quality of life.
“The single best method we’ve ever used in the care of plantar fasciopathy has been Wharton’s jelly umbilical cord tissue allografts,” said Babak Baravarian, DPM, senior author of the study and Director of Foot & Ankle Research at University Foot & Ankle Institute.
Study Findings
In this small observational cohort (n=9; mean age 73), patients demonstrated an overall trend toward improvement across pain, function, and quality-of-life measures over the 90-day follow-up period. Reported outcomes included:
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected] Scientific / Publication Contact Naomi Lambert
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected] SOURCE Regenative Labs
- 60.98% mean improvement in Numeric Pain Rating Scale (NPRS) scores from baseline to Day 90
- 49.55% mean improvement in WOMAC Total scores from baseline to Day 90, with statistically significant improvements observed across total and subscale measures
- No adverse reactions reported during the observational period
- Design: Observational case series with follow-ups at baseline, 30 days, and 90 days
- Population: 9 patients with plantar fasciopathy (mean age 73; 5 male, 4 female)
- Eligibility: Patients had failed at least three months of conservative therapy prior to allograft application
- Intervention: Single ultrasound-guided application of 2 cc of 150 mg/mL Wharton’s jelly connective tissue allograft into areas of confirmed degeneration
- Outcomes Measured: NPRS, WOMAC, and Quality-of-Life Scale (QOLS)
- Safety: No adverse reactions reported
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected] Scientific / Publication Contact Naomi Lambert
Regenative Labs
Phone: +1-800-891-3452
Email: [email protected] SOURCE Regenative Labs

Source link














Leave a Reply